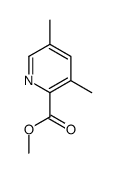
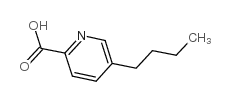
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |

![3-butyl-9,9-dimethyl-7,11-dioxo-8,10-dioxa-1-azaspiro[5.5]undec-3-en-1-yl 4-methylbenzenesulfonate结构式](https://image.chemsrc.com/caspic/044/1027940-16-4.png)