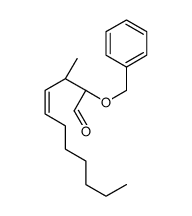
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
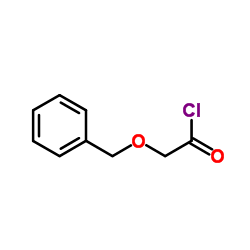

![[(Z)-dec-2-en-4-yl] 2-phenylmethoxyacetate结构式](https://image.chemsrc.com/caspic/315/102616-19-3.png)