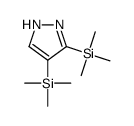
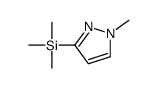
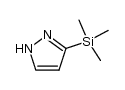
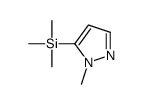
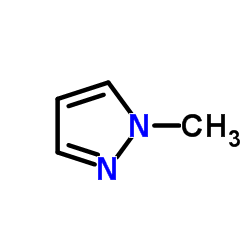
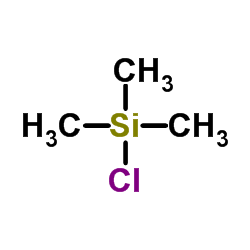
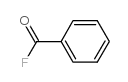
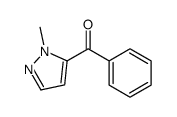
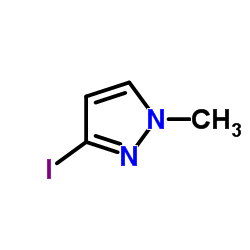
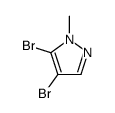
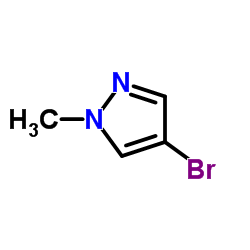
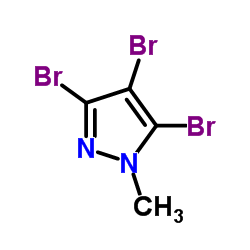
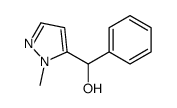
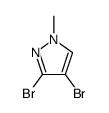
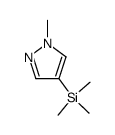
|
~7% |
|
~68% |
|
~4% |
|
~50% |
|
~62% |
|
~45% |
|
~92% |
|
~45% |
|
~% |
|
~33% |
|
~% |
|
~% |
|
~72% |
|
~0% |
|
~53% |