|
~75% |
|
~85% |
|
~% |
|
~% |
|
~% |
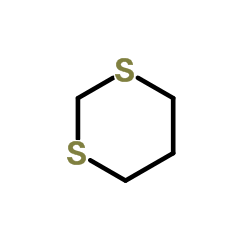
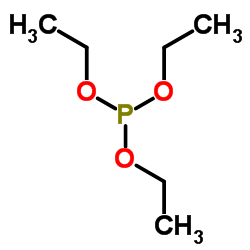
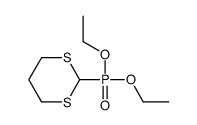
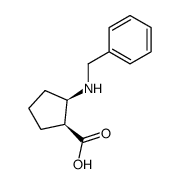
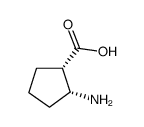
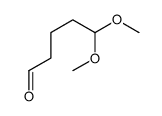
![(+)-2-(5,5-dimethoxy-pentylidene)-[1S,3S]-1,3-dioxo-dithiane结构式](https://image.chemsrc.com/caspic/077/420123-95-1.png)
![(-)-4-aza-3-oxa-4-benzyl-2-spiro(1',3'-dithiane-[1'S,3'S]-1',3'-dioxo)-[5R,9S]-bicyclo[3.3.0] octane结构式](https://image.chemsrc.com/caspic/039/420123-96-2.png)