|
~82% |
|
~% |
|
~% |
|
~90% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |

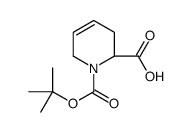

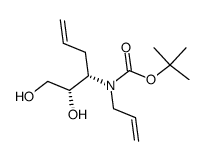
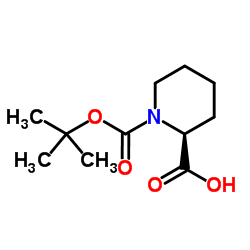
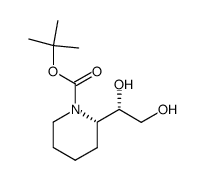
![(S)-N-tert-butoxycarbonyl-1-[(S)-1',2',3',6'-tetrahydropyridin-2-yl]-ethan-1,2-diol结构式](https://image.chemsrc.com/caspic/136/417726-33-1.png)