|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~% |

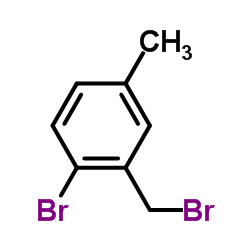

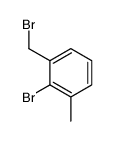
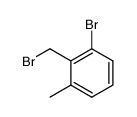
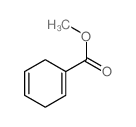

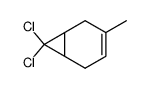
![(7,7-dichlorobicyclo[4.1.0]hept-3-en-3-yl)methanol结构式](https://image.chemsrc.com/caspic/303/75366-08-4.png)
![methyl 7,7-dichlorobicyclo[4.1.0]hept-3-ene-3-carboxylate结构式](https://image.chemsrc.com/caspic/320/75444-70-1.png)

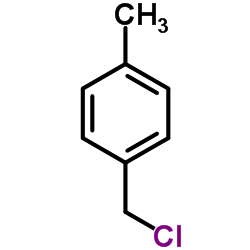

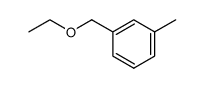


![N-[(3-methylphenyl)methyl]aniline结构式](https://www.chemsrc.com/extcaspic/286/75366-13-1.png)