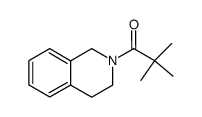
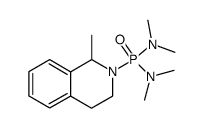
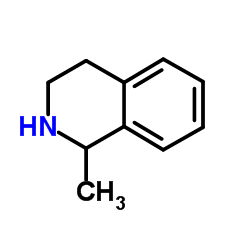
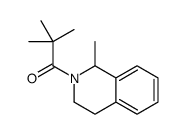
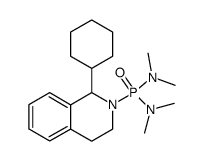
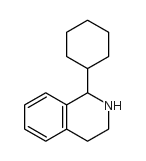
|
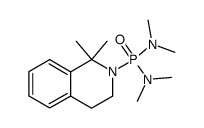
~75% |
|
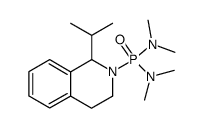
~82% |
|
~% |
|
~% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |