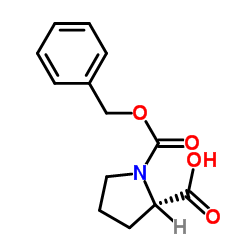
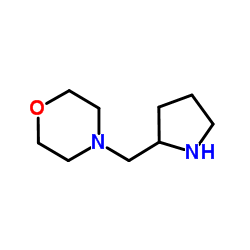
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![(S)-4-[N-(benzyloxycarbonyl)prolyl]morpholine结构式](https://image.chemsrc.com/caspic/216/66165-42-2.png)
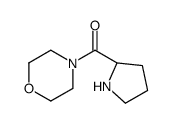
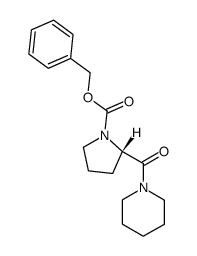
![1-[(2S)-吡咯烷甲基]哌啶结构式](https://image.chemsrc.com/caspic/187/65921-41-7.png)