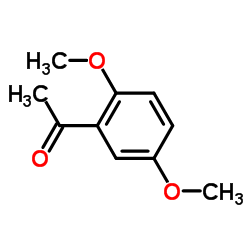
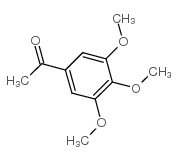
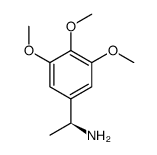
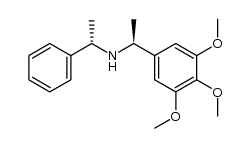
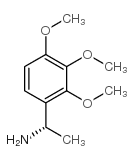
|
~87% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~95% |
|
~63% |
|
~% |
|
~% |
|
~92% |
|
~60% |
|
~% |
|
~% |
|
~92% |
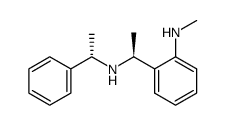
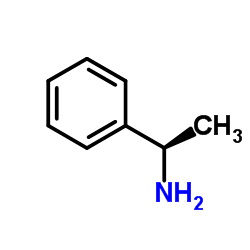
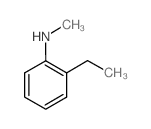
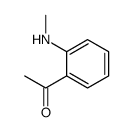
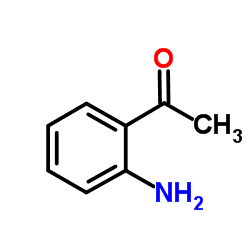
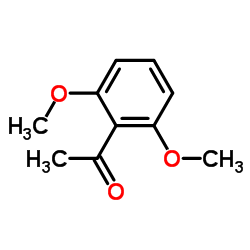
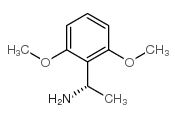
![(S)-1-(2,5-二甲氧基苯基)-N-[(S)-1-苯基乙基]乙胺结构式](https://image.chemsrc.com/caspic/161/128113-78-0.png)