|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |


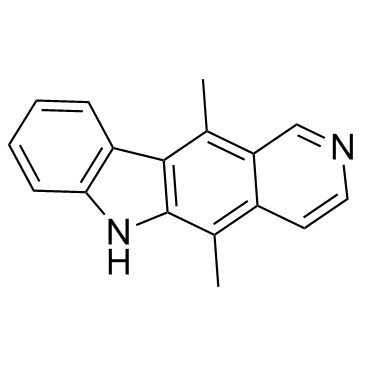

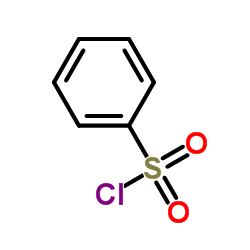
![4-(benzenesulfonyl)-1,3-dimethylfuro[3,4-b]indole结构式](https://image.chemsrc.com/caspic/458/89241-38-3.png)
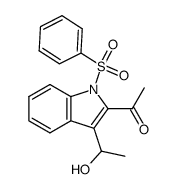
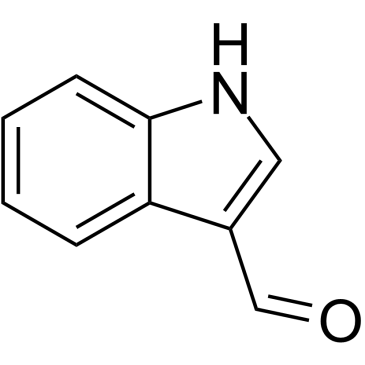
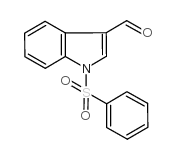
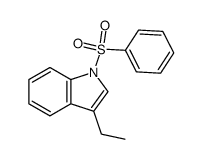
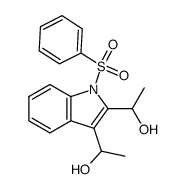
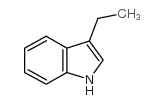

![4-(benzenesulfonyl)-1,3-dimethyl-3H-furo[3,4-b]indol-1-ol结构式](https://image.chemsrc.com/caspic/420/89241-39-4.png)