|
~84% |
|
~73% |
|
~67% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~80% |
|
~% |
|
~41% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
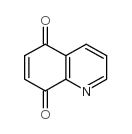

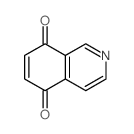
![benzo[g]quinoline-5,10-dione结构式](https://image.chemsrc.com/caspic/110/3712-09-2.png)
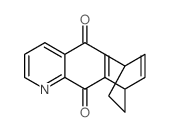

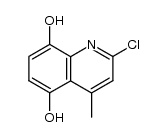
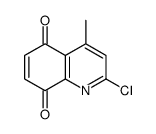
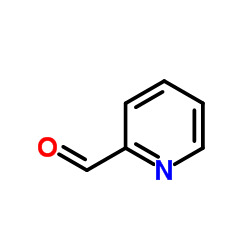
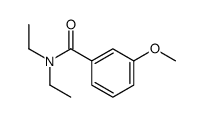
![9-methoxybenzo[g]quinoline-5,10-dione结构式](https://image.chemsrc.com/caspic/241/90381-59-2.png)
![6-methoxybenzo[g]isoquinoline-5,10-dione结构式](https://image.chemsrc.com/caspic/310/90381-62-7.png)
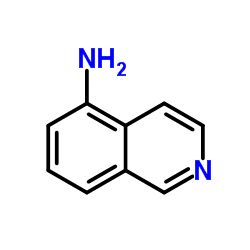
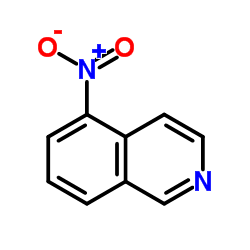
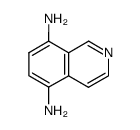
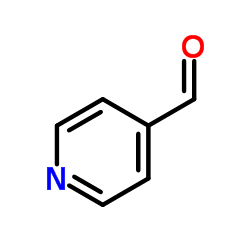
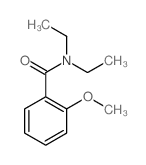
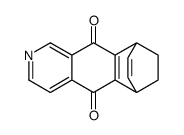
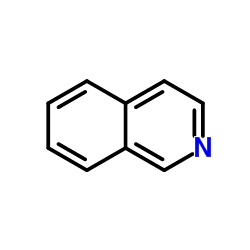
![苯并[g]异喹啉-5,10-二酮结构式](https://image.chemsrc.com/caspic/333/46492-08-4.png)