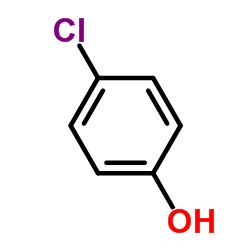
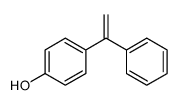
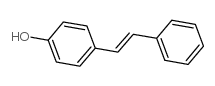
|
~13% |
|
~% |
|
~82% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
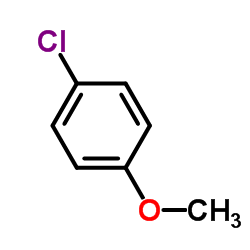
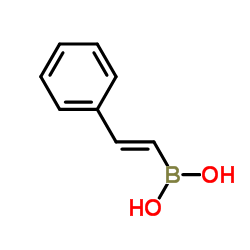
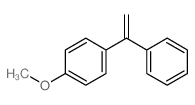
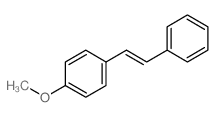
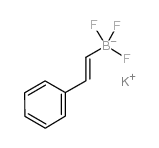
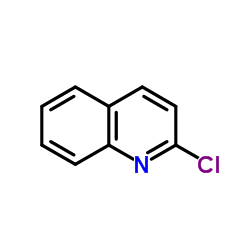
![Quinoline,2-[(1E)-2-phenylethenyl]结构式](https://image.chemsrc.com/caspic/022/38101-69-8.png)
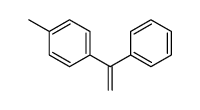
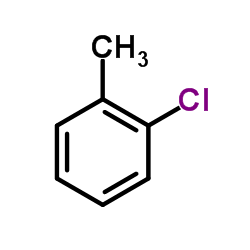
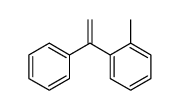
![1-methyl-2[(1Z)-2-phenylethenyl]benzene结构式](https://image.chemsrc.com/caspic/305/53423-25-9.png)
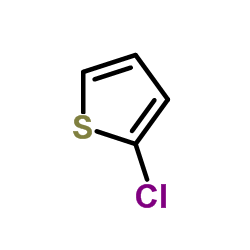
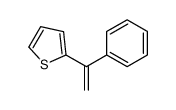
![2-[(E)-2-苯乙烯基]噻吩结构式](https://image.chemsrc.com/caspic/337/3783-65-1.png)