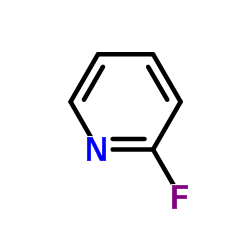
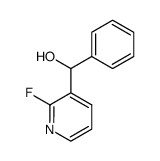
|
~90% |
|
~88% |
|
~87% |
|
~% |
|
~% |
|
~% |
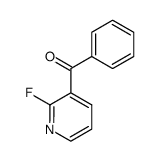
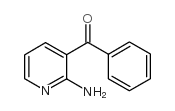
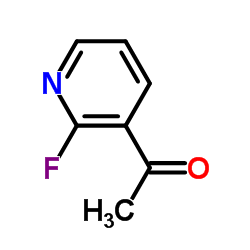
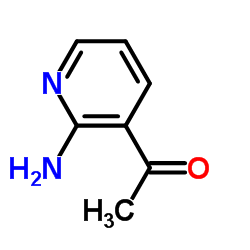

![[2-(methylamino)pyridin-3-yl]-phenylmethanone结构式](https://image.chemsrc.com/caspic/081/79574-77-9.png)