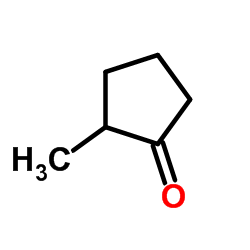
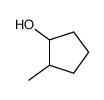
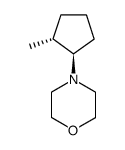
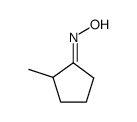
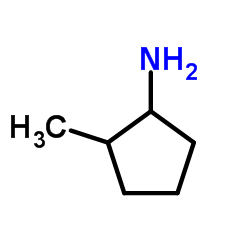
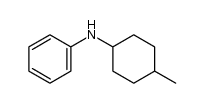
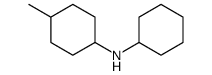
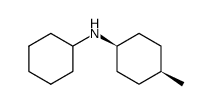
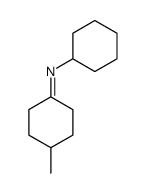
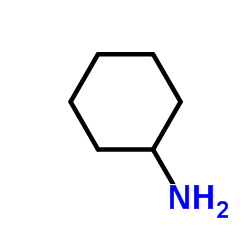
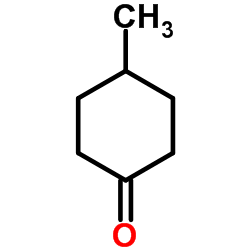
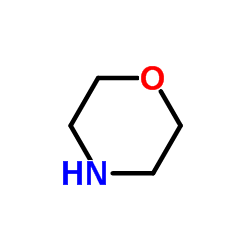
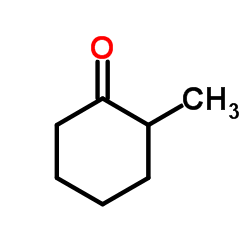
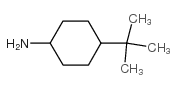
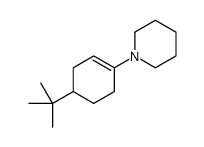
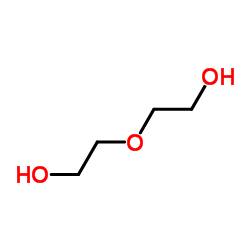
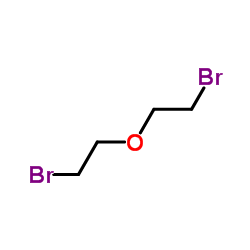
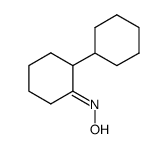
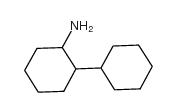
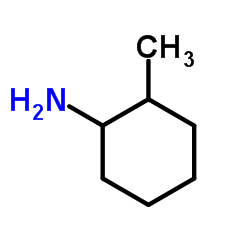
|
~% |
|
~45% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~74% |
|
~95% |
|
~% |
|
~55% |
|
~32% |
|
~56% |
|
~60% |