|
~68% |
|
~72% |
|
~72% |
|
~61% |
|
~55% |
|
~75% |
|
~65% |
|
~74% |
|
~66% |
|
~26% |
|
~75% |
|
~77% |
|
~63% |
|
~72% |
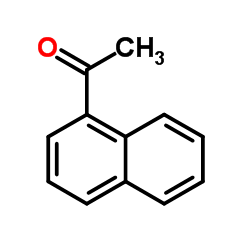
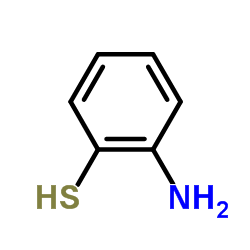
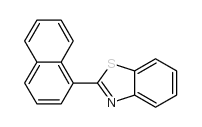
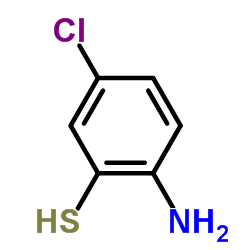

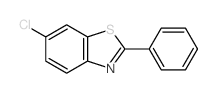
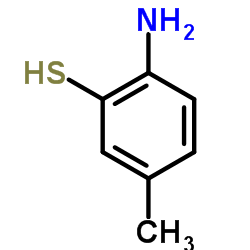
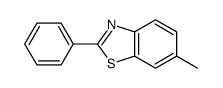
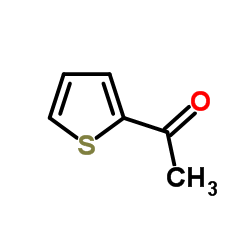
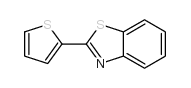
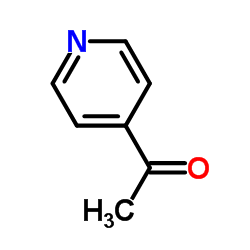
![2-(吡啶-4-基)苯并[d]噻唑结构式](https://image.chemsrc.com/caspic/216/2295-38-7.png)
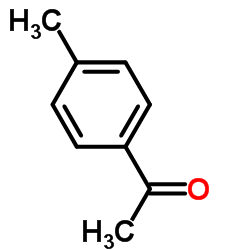

![6-甲氧基-2-(对甲苯基)苯并[d]噻唑结构式](https://image.chemsrc.com/caspic/432/101078-51-7.png)


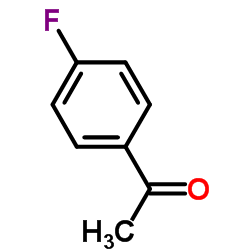



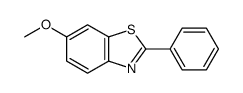
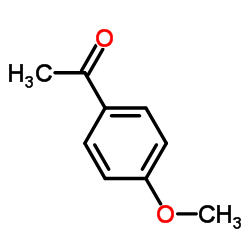
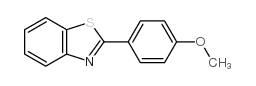
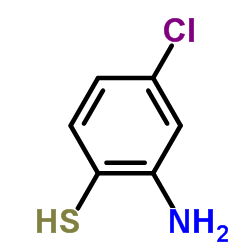
![5-氯-2-苯基苯并[d]噻唑结构式](https://image.chemsrc.com/caspic/224/952-16-9.png)