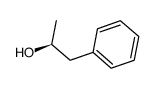
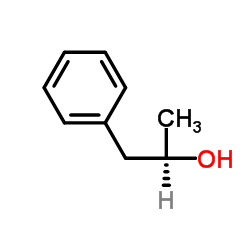
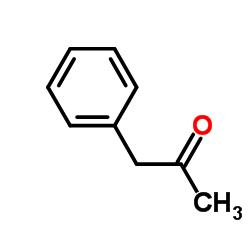
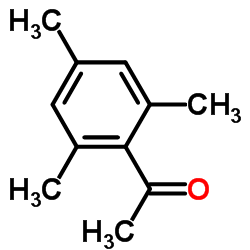
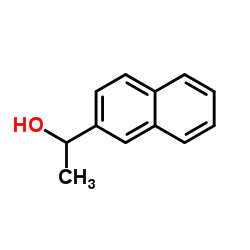
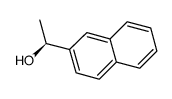
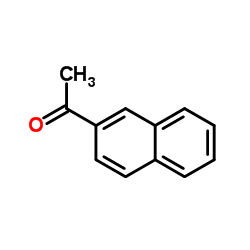
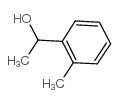
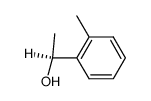
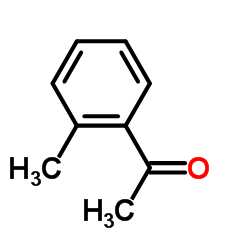
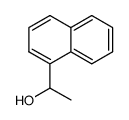
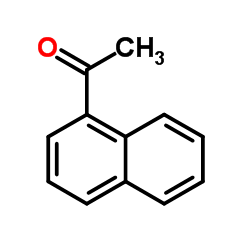
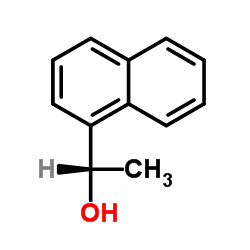
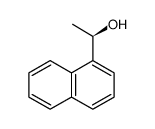
|
~0% |
|
~55% |
|
~0% |
|
~0% |
|
~0% |
|
~51% |
|
~0% |
|
~0% |
|
~60% |