|
~86% |
|
~% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~90% |
|
~82% |
|
~74% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
![(1R)-1-[2-(methoxymethoxy)phenyl]ethanamine结构式](https://image.chemsrc.com/caspic/282/368447-78-3.png)

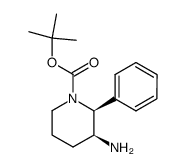
![3-[(2-METHOXYBENZYL)AMINO]-2-PHENYL-PIPERIDINE结构式](https://image.chemsrc.com/caspic/312/136982-36-0.png)
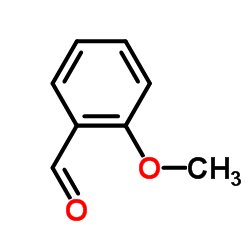
![(S)-3-[(E)-Methoxyimino]-2-phenyl-piperidine-1-carboxylic acid tert-butyl ester结构式](https://image.chemsrc.com/caspic/356/500021-57-8.png)
![(2S)-2-[({(1R)-1-[3-(methylsulfanyl)phenyl]ethyl}amino)oxy]-2-phenylethanol结构式](https://image.chemsrc.com/caspic/358/368447-76-1.png)
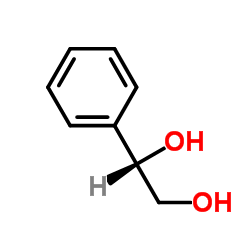
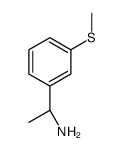
![3-(methylsulfanyl)benzaldehyde (E)-O-[(1S)-2-hydroxy-1-phenylethyl]oxime结构式](https://image.chemsrc.com/caspic/096/368447-70-5.png)
![(2S)-2-phenyl-2-({[(1R)-1-phenylethyl]amino}oxy)ethanol结构式](https://image.chemsrc.com/caspic/058/368447-71-6.png)
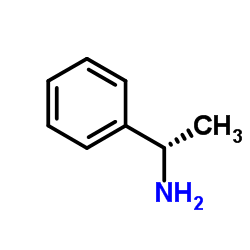
![(2S)-2-({[(1R)-1-(4-methylphenyl)ethyl]amino}oxy)-2-phenylethanol结构式](https://image.chemsrc.com/caspic/020/368447-72-7.png)

![(2S)-2-({[(1R)-1-(3-methoxyphenyl)ethyl]amino}oxy)-2-phenylethanol结构式](https://image.chemsrc.com/caspic/444/368447-74-9.png)
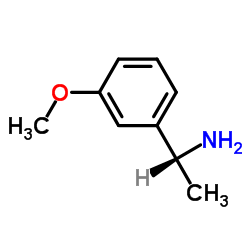
![(2S)-2-({[(1R)-1-(2-methoxyphenyl)ethyl]amino}oxy)-2-phenylethanol结构式](https://image.chemsrc.com/caspic/482/368447-73-8.png)

![(2S)-2-[({(1R)-1-[2-(methoxymethoxy)phenyl]ethyl}amino)oxy]-2-phenylethanol结构式](https://image.chemsrc.com/caspic/396/368447-75-0.png)
![(1R)-2-({[(1S)-2,2-dimethyl-1-phenylpropyl]amino}oxy)ethanol结构式](https://image.chemsrc.com/caspic/133/757195-26-9.png)
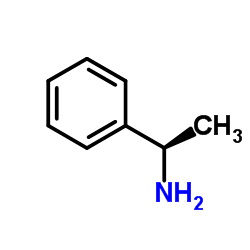

![(1R)-2-({[(1S)-2,2-dimethyl-1-phenylpropyl]amino}oxy)ethanol结构式](https://image.chemsrc.com/caspic/078/757195-32-7.png)
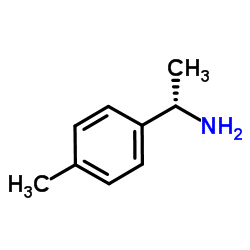


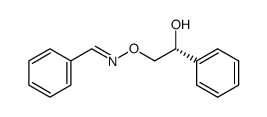
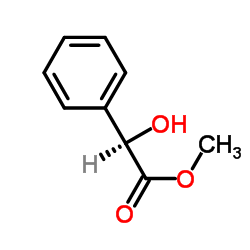
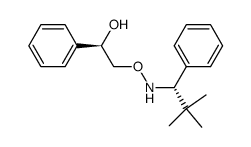
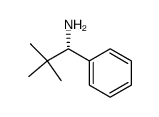
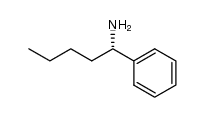
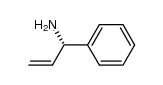
![(1R)-2-[({(1S)-1-[2-(methoxymethoxy)phenyl]ethyl}amino)oxy]-1-phenylethanol结构式](https://image.chemsrc.com/caspic/133/757195-48-5.png)
![(1S)-1-[2-(methoxymethoxy)phenyl]ethanamine结构式](https://image.chemsrc.com/caspic/240/757195-50-9.png)
![(1R)-1-phenyl-2-({[(1S)-1-phenyl-2-propenyl]amino}oxy)ethanol结构式](https://image.chemsrc.com/caspic/447/757195-40-7.png)
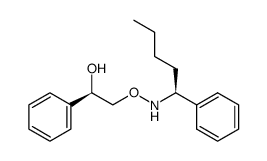
![(1R)-1-phenyl-2-({[(1S)-1-phenylpentyl]amino}oxy)ethanol结构式](https://image.chemsrc.com/caspic/047/757195-28-1.png)