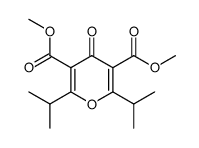
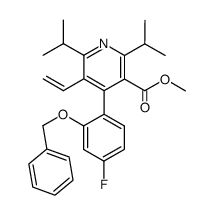
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
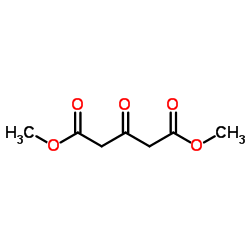
![4-[4-氟-2-(苯基甲氧基)苯基]-5-甲酰基-2,6-双(1-甲基乙基)-3-吡啶羧酸甲酯结构式](https://image.chemsrc.com/caspic/087/618892-25-4.png)
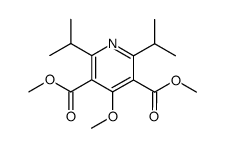
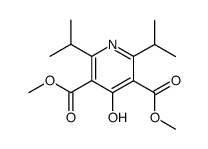
![3,5-Pyridinedicarboxylic acid, 4-[4-fluoro-2-(phenylmethoxy)phenyl]-2,6-bis(1-methylethyl)-, 3,5-dimethyl ester结构式](https://image.chemsrc.com/caspic/365/470717-47-6.png)

![5-乙烯基-4-[4-氟-2-(苯基甲氧基)苯基]-2,6-双(1-甲基乙烯基)-3-吡啶甲醛结构式](https://image.chemsrc.com/caspic/163/202858-60-4.png)