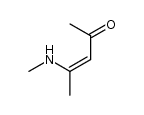
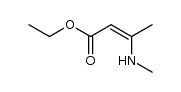
|
~23%
详细
|
|
~10% |
|
~6% |
|
~33% |
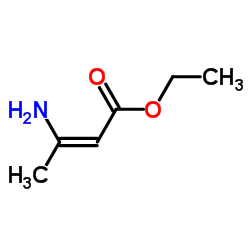
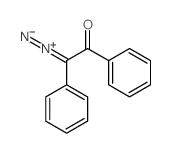
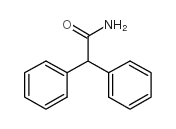

![Fluoral-P(=4-氨基-3-戊烯-2-酮)[用于醛的荧光试剂]结构式](https://image.chemsrc.com/caspic/096/1118-66-7.png)