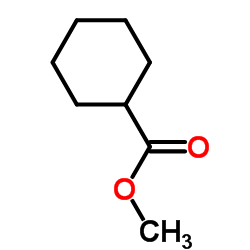
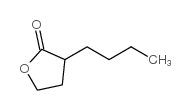
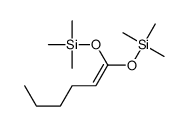
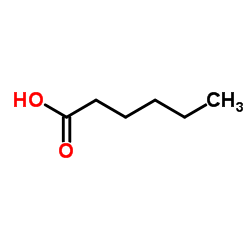
|
~54% |
|
~64% |
|
~% |
|
~69% |
|
~79% |
|
~% |
|
~% |
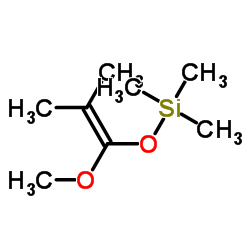
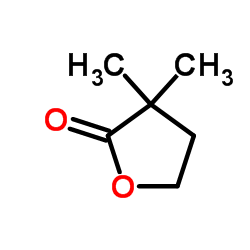
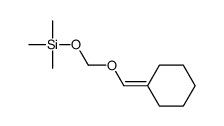
![2-氧杂螺[4.5]癸-1-酮结构式](https://image.chemsrc.com/caspic/423/4420-11-5.png)