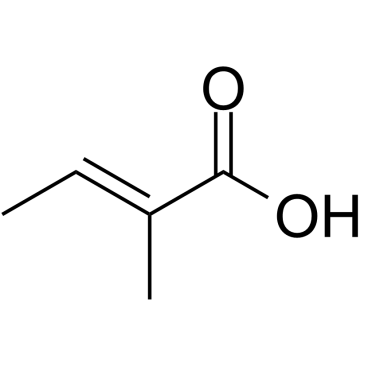
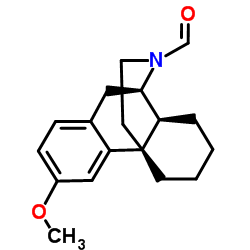
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |


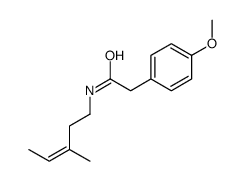
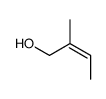

![(Z)-2-Formyl-1-[(4-methoxyphenyl)methylene]-1,2,3,4,5,6,7,8-octahydroisoquinoline结构式](https://image.chemsrc.com/caspic/296/116172-20-4.png)
-isoquinoline-2-carbaldehyde结构式](https://image.chemsrc.com/caspic/384/29144-31-8.png)
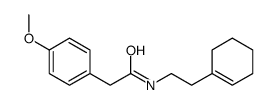
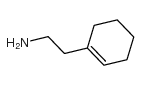
![3,4,5,6,7,8-hexahydro-1-[(4-methoxyphenyl)methyl]isoquinoline结构式](https://image.chemsrc.com/caspic/433/51072-35-6.png)