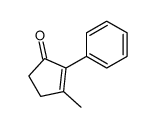
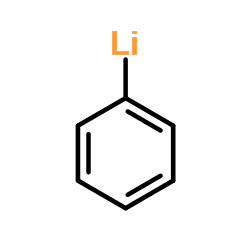
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![6-Oxabicyclo[3.1.0]hexan-2-one,5-methyl结构式](https://image.chemsrc.com/caspic/285/17024-44-1.png)
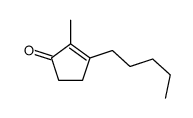
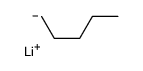

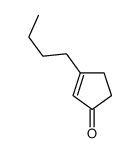
![6-Methyl-7-oxabicyclo[4.1.0]heptan-2-one结构式](https://image.chemsrc.com/caspic/479/21889-89-4.png)
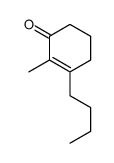
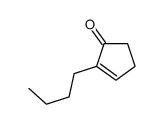
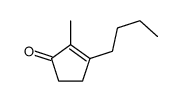
![7-氧杂二环[4.1.0]庚烷-2-酮结构式](https://image.chemsrc.com/caspic/454/6705-49-3.png)