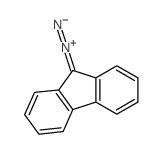
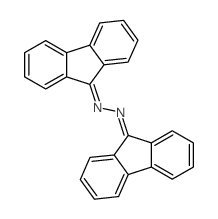
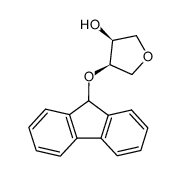
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~76% |
![1-[diazo-(4-methylphenyl)methyl]-4-methylbenzene结构式](https://image.chemsrc.com/caspic/450/1143-91-5.png)
![N-[bis(4-methylphenyl)methylideneamino]-1,1-bis(4-methylphenyl)methanimine结构式](https://image.chemsrc.com/caspic/112/5895-68-1.png)
![1,4-anhydro-2-O-[bis(4-methylphenyl)methyl]-DL-erythritol结构式](https://image.chemsrc.com/caspic/180/82198-54-7.png)

![methyl 4,6-O-benzylidene-3-O-[bis(4-methylphenyl)methyl]-α-D-mannopyranoside结构式](https://image.chemsrc.com/caspic/053/82185-98-6.png)
![3-O-[bis(4-methylphenyl)methyl]-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose结构式](https://image.chemsrc.com/caspic/129/82185-96-4.png)