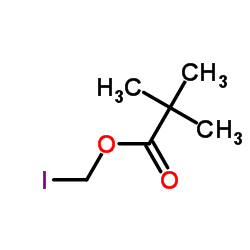
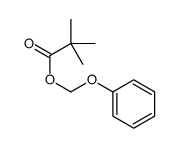
|
~71% |
|
~70% |
|
~% |
|
~66% |
|
~83% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

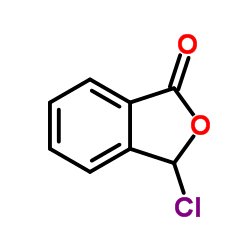
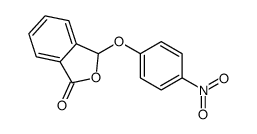

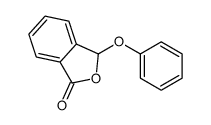
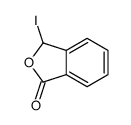
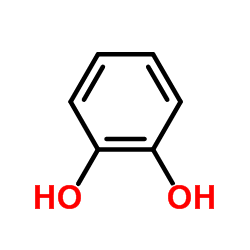
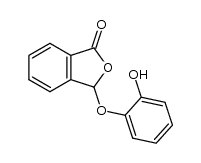
![3-[2-[(3-oxo-1H-isobenzofuran-1-yl)oxy]phenoxy]-3H-isobenzofuran-1-one结构式](https://image.chemsrc.com/caspic/164/87116-23-2.png)