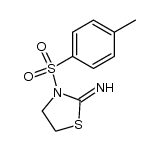
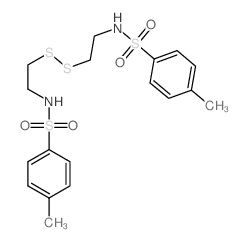
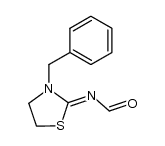
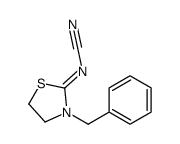
|
~% |
|
~29% |
|
~67% |
|
~2% |
|
~% |
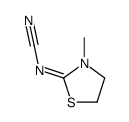

![[3-(4-methylphenyl)sulfonyl-1,3-thiazolidin-2-ylidene]cyanamide结构式](https://image.chemsrc.com/caspic/172/97097-66-0.png)