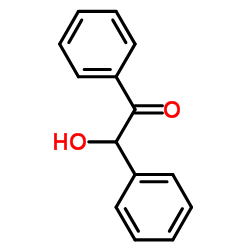
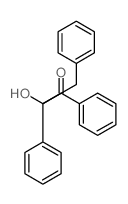
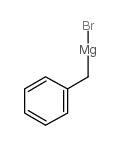
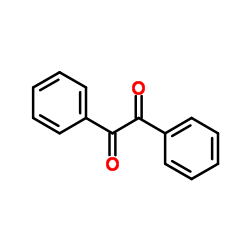
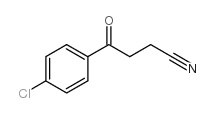
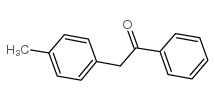
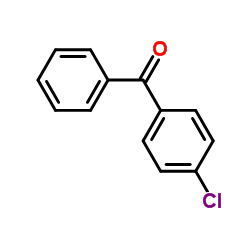
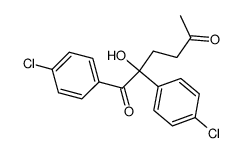
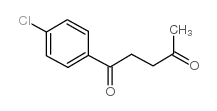
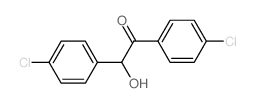
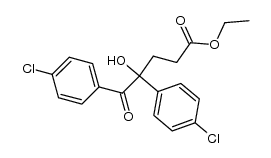
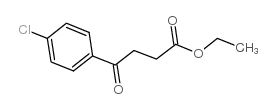
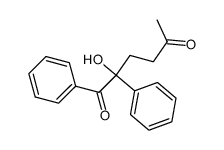
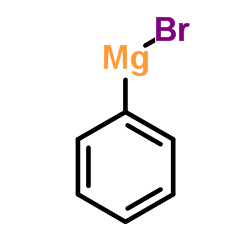
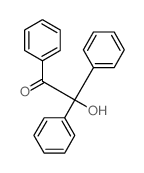
|
~78% |
|
~92% |
|
~% |
|
~86% |
|
~% |
|
~10% |
|
~71% |
|
~98% |
|
~% |
|
~% |
|
~96% |
|
~96% |
|
~58% |