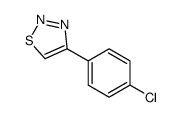
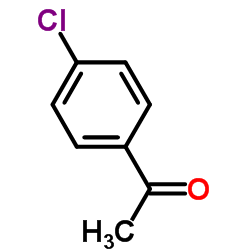
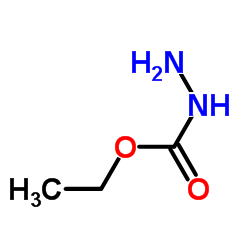
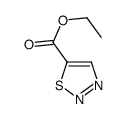
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

![[1,2,3]噻二唑-4-羰基氯结构式](https://image.chemsrc.com/caspic/417/4100-17-8.png)

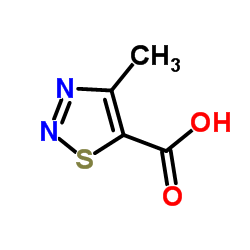
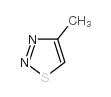
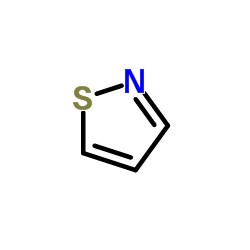


![ethyl N-[1-(4-chlorophenyl)ethylideneamino]carbamate结构式](https://image.chemsrc.com/caspic/415/25445-82-3.png)