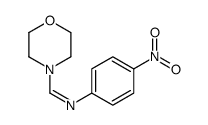
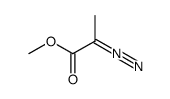
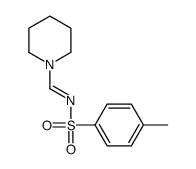
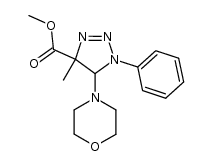
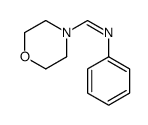
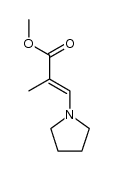
|
~60% |
|
~90% |
|
~75% |
|
~87% |