Discovery of potent and selective BRD4 inhibitors capable of blocking TLR3-induced acute airway inflammation
Zhiqing Liu, Bing Tian, Haiying Chen, Pingyuan Wang, Allan R. Brasier, Jia Zhou
文献索引:10.1016/j.ejmech.2018.04.006
全文:HTML全文
摘要
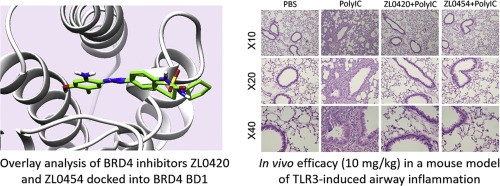
A series of diverse small molecules have been designed and synthesized through structure-based drug design by taking advantage of fragment merging and elaboration approaches. Compounds ZL0420 (28) and ZL0454 (35) were identified as potent and selective BRD4 inhibitors with nanomolar binding affinities to bromodomains (BDs) of BRD4. Both of them can be well docked into the acetyl-lysine (KAc) binding pocket of BRD4, forming key interactions including the critical hydrogen bonds with Asn140 directly and Tyr97 indirectly via a H2O molecule. Both compounds 28 and 35 exhibited submicromolar potency of inhibiting the TLR3-dependent innate immune gene program, including ISG54, ISG56, IL-8, and Groβ genes in cultured human small airway epithelial cells (hSAECs). More importantly, they also demonstrated potent efficacy reducing airway inflammation in a mouse model with low toxicity, indicating a proof of concept that BRD4 inhibitors may offer the therapeutic potential to block the viral-induced airway inflammation.
|
New lipophilic isoniazid derivatives and their 1,3,4-oxadiaz...
2018-04-10 [10.1016/j.ejmech.2018.04.017] |
|
Discovery of new benzensulfonamide derivatives as tripedal S...
2018-04-04 [10.1016/j.ejmech.2018.03.053] |
|
2-Benzylpiperazine: A new scaffold for potent human carbonic...
2018-04-03 [10.1016/j.ejmech.2018.04.002] |
|
Anti-leishmanial click modifiable thiosemicarbazones: Design...
2018-04-03 [10.1016/j.ejmech.2018.04.003] |
|
Design and synthesis of BPR1K653 derivatives targeting the b...
2018-04-03 [10.1016/j.ejmech.2018.03.064] |