Effective degradation of phenol via Fenton reaction over CuNiFe layered double hydroxides
Hao Wang, Mengmeng Jing, Yan Wu, Weiliang Chen, Yao Ran
文献索引:10.1016/j.jhazmat.2018.03.053
全文:HTML全文
摘要
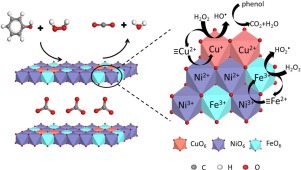
A series of CuNiFe layered double hydroxides (LDHs) with various Cu/Ni molar ratios were synthesized as catalysts for Fenton degradation of phenol. It is found that Cu+, Cu2+, Ni2+, Ni3+ and Fe3+ are present on LDHs, owing to an electron transfer from Ni2+ to Cu2+ via metal-oxo-metal bridges. At lower Cu/Ni ratios, the highly dispersed MO6 octahedra and the electron donation effect of Ni facilitate such electron transfer and thus increase the percentage of Cu+. The catalytic activity increases with the decrease in Cu/Ni ratio. The most active Cu0.5Ni2.5Fe LDH can mineralize 98.9% phenol at ambient pH and less excessive H2O2 dosage (MH2O2/Mphenol = 37). Even at the H2O2 dosage close to the theoretical value, around 90% phenol can be mineralized. The structure-activity correlation indicates Cu+ which can readily react with H2O2 to produce hydroxyl radicals may dominate the reaction. The regeneration of Cu+ could be achieved by the electron transfer between Cu2+ and Ni2+ in LDHs. Moreover, Fe3+ can also act as Fenton-like active sites. The special structure of CuNiFe LDHs could offer surface-enriched and easily regenerated Cu+ species, leading to the complete mineralization of phenol and the efficient use of H2O2.
|
Humic acid attenuation of silver nanoparticle toxicity by io...
2018-04-11 [10.1016/j.jhazmat.2018.04.019] |
|
In Situ Preparation of Highly Stable Polyaniline/W18O49 Hybr...
2018-04-10 [10.1016/j.jhazmat.2018.04.005] |
|
Effects of Trifluralin on the Soil Microbial Community and Fu...
2018-04-10 [10.1016/j.jhazmat.2018.04.012] |
|
Effects of different oxyanions in solution on the precipitat...
2018-04-09 [10.1016/j.jhazmat.2018.04.016] |
|
Comparison of constant, pulsed, incremental and decremental ...
2018-04-08 [10.1016/j.jhazmat.2018.04.002] |