Total synthesis of (±)-(1β,4β,4aβ,8aα)-4,8a-dimethyl-octahydro-naphthalene-1,4a(2H)-diol
Qingyin Liu, Li Han, Bing Qin, Yu Mu, Peipei Guan, Songyao Wang, Xueshi Huang
文献索引:10.1039/C8QO00225H
全文:HTML全文
摘要
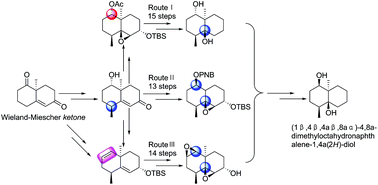
The first total synthesis of (±)-(1β,4β,4aβ,8aα)-4,8a-dimethylocta-hydronaphthalene-1,4a(2H)-diol (1), a degraded sesquiterpene isolated from a fermentation broth of Streptomyces albolongus, has been achieved via three different synthetic approaches (13–15 steps) starting from racemic Wieland–Miescher ketone (2). The configuration of hydroxyl groups at C-1 and C-4a in 1 was availably managed using the Mitsunobu reaction and stereo- and regioselective epoxidation. Moreover, the syntheses of configuration isomers 12, 17, 45 and 54 were also described in the present study.
|
Design of Rigid Cyclic Tripyrrins: The Importance of Intermo...
2018-04-11 [10.1039/C8QO00313K] |
|
Enantioselective indium(I)-catalyzed [4 + 2] annulation of a...
2018-04-09 [10.1039/C8QO00319J] |
|
Controllable synthetic ion channels
2018-04-09 [10.1039/C8QO00287H] |
|
α,β-Diaryl unsaturated ketones via palladium-catalyzed ring-...
2018-04-04 [10.1039/C8QO00241J] |
|
The stabilizing effect of the transient imine directing grou...
2018-04-02 [10.1039/C8QO00094H] |