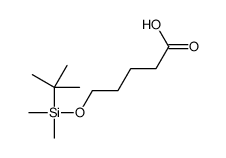
|
~% |
|
~% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~59% |
|
~% |
|
~17% |
|
~80% |
|
~% |
|
~% |

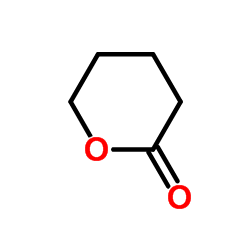
![3-oxabicyclo[3.2.0]hept-6-ene-2,4-dione结构式](https://image.chemsrc.com/caspic/183/10374-07-9.png)
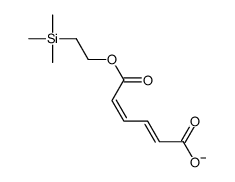
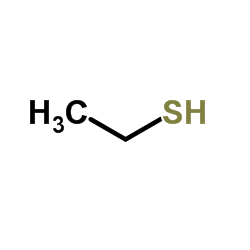
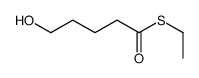
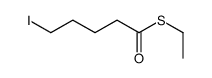
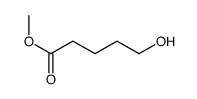
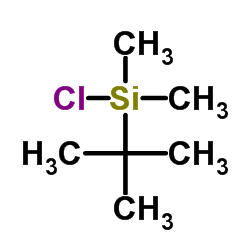
![5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-Pentanoicacidmethylester结构式](https://image.chemsrc.com/caspic/312/87729-38-2.png)