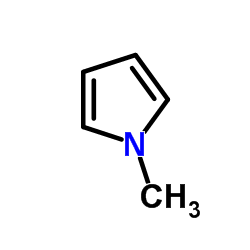
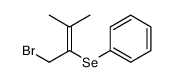
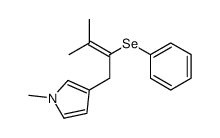
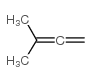
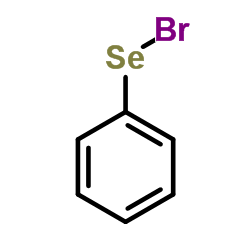
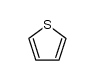
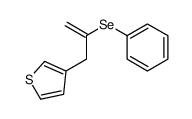
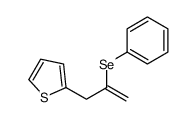
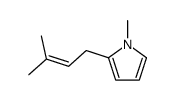
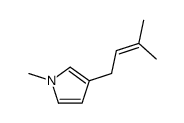
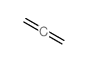
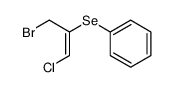
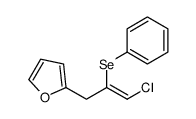
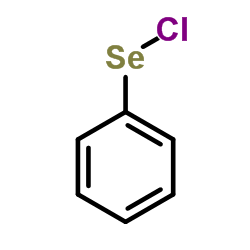
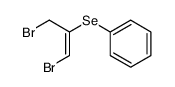
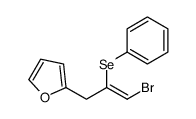
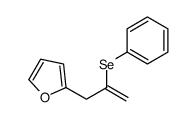
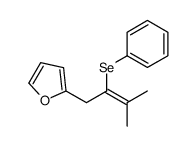
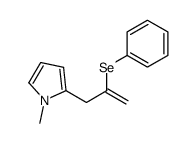
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~62% |
|
~% |
|
~62% |
|
~% |
|
~62% |
|
~% |
|
~% |
|
~% |
|
~60% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |