Aromatic polyesters derived from 5,5′-disubstituted BIS(m-phenylene) crown) ethers
Harry W. Gibson, Devdatt S. Nagvekar, Norimitsu Yamaguchi, Yadollah Delaviz, Jason W. Jones, Peter Balanda, Abaneshwar Prasad, Herve’ Marand
文献索引:10.1016/j.polymer.2018.03.031
全文:HTML全文
摘要
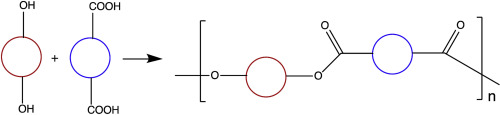
A new monomer of the AB type, 5-carboxy-1,3-phenylene-5′-hydroxy-1′,3′-phenylene-32-crown-10 (5), was synthesized. Direct polymerizations of bis(5-hydroxy-1,3-phenylene)-32-crown-10 (13), an AA diphenolic macrocyclic monomer, with BB diacid monomer bis(5-carboxy-1,3-phenylene)-26-crown-8 (14) and its larger analog bis(5-carboxy-1,3-phenylene)-32-crown-10 (16) were accomplished via the Higashi method. The resultant polyesters 15 and 17, which both contain two macrocycles per repeat unit, were soluble in common organic solvents such as chloroform and tetrahydrofuran, amorphous in nature and possessed high thermal stability (5% weight loss > 370 °C in air). Main chain poly (ester crown ether)s 18 and 19 based on bis(5-carboxy-1,3-phenylene)-32-crown-10 (16), and hydroquinone (HQ) and biphenol (BP), respectively, were synthesized; these two polyesters also possess high thermal stability (5% weight loss > 386 °C in air) and are amorphous. These polymacrocycles are suitable polytopic hosts for complexation with 4,4′-bipyridinium derivatives, as demonstrated by the binding of paraquat diol (21a) by polyester 20 with Ka = 2.0 (±0.7) x 102 M−1 (23 °C, 4:6 w:w CD3CN:CDCl3). Threading of the cyclic units in these polyesters by linear polymers such as polystyrene and PMMA leads to compatiblized blends; this methodology represents a new approach to compatibilization.
|
Effect of hydrogen bonding on the liquid crystalline behavio...
2018-04-10 [10.1016/j.polymer.2018.04.028] |
|
Thermal transitions in semi-crystalline polymer thin films s...
2018-04-04 [10.1016/j.polymer.2018.04.017] |
|
Chemical recycling of poly(bisphenol A carbonate): 1,5,7-Tri...
2018-04-04 [10.1016/j.polymer.2018.04.015] |
|
Simultaneous WAXS/SAXS study on semi-crystalline Poly(ethyle...
2018-04-04 [10.1016/j.polymer.2018.04.018] |
|
Gallol-containing homopolymers and block copolymers: ROMP sy...
2018-04-04 [10.1016/j.polymer.2018.04.016] |