|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~26% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
|
~37% |
|
~61% |
|
~63% |
|
~% |
|
~% |


![1-[4-(1,3-dithian-2-ylidene)butyl]pyrrolidine-2,5-dione结构式](https://image.chemsrc.com/caspic/176/89556-85-4.png)
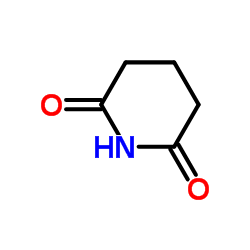
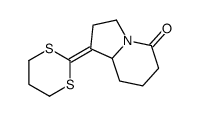
![1-[3-(1,3-dithian-2-ylidene)propyl]piperidine-2,6-dione结构式](https://image.chemsrc.com/caspic/138/89556-86-5.png)

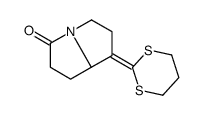
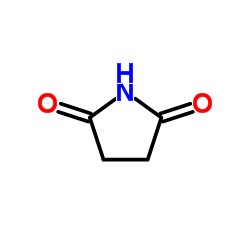
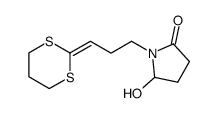
![1-[3-(1,3-dithian-2-ylidene)propyl]pyrrolidine-2,5-dione结构式](https://image.chemsrc.com/caspic/247/83177-75-7.png)
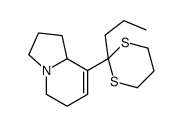
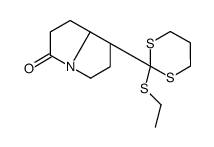
![1-Aza-4-(1,3-dithian-2-yl)-bicyclo[3.3.0]octan-8-one结构式](https://image.chemsrc.com/caspic/123/83177-78-0.png)

![1-[4-(1,3-dithian-2-ylidene)butyl]piperidine-2,6-dione结构式](https://image.chemsrc.com/caspic/100/89556-87-6.png)
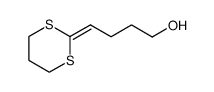
![5-([1,3]dithian-2-ylidene)pentan-1-ol结构式](https://image.chemsrc.com/caspic/377/347191-48-4.png)
![9-(1,3-dithian-2-ylidene)-2,5,6,7,8,9a-hexahydro-1H-pyrrolo[1,2-a]azepin-3-one结构式](https://image.chemsrc.com/caspic/083/89556-92-3.png)
![1-[5-(1,3-dithian-2-ylidene)pentyl]pyrrolidine-2,5-dione结构式](https://image.chemsrc.com/caspic/062/89556-88-7.png)