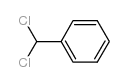
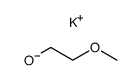
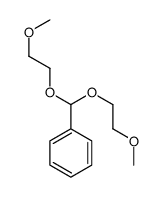
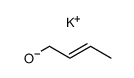
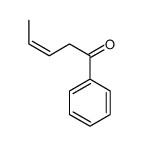
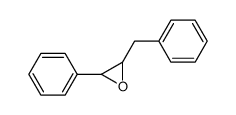
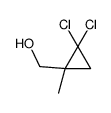
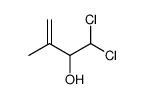
|
~5%
详细
|
|
~5% |
|
~39% |
|
~% |
|
~90% |
|
~% |