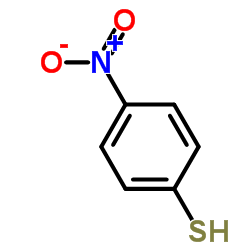
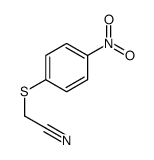
|
~% |
|
~% |
|
~% |
|
~% |
|
~77% |



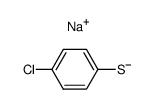


![[cyano-(4-methoxyphenyl)sulfanylmethyl] acetate结构式](https://image.chemsrc.com/caspic/123/63923-63-7.png)