Double Gold Activation of 1-Ethynyl-2-(Phenylethynyl)Benzene Toward 5-exo-dig and 6-endo-dig Cyclization Reactions
Nery Villegas-Escobar, Mie Højer Larsen (née Vilhelmsen), Soledad Gutiérrez-Oliva, A. Stephen K. Hashmi, Alejandro Toro-Labbé
文献索引:10.1002/chem.201701595
全文:HTML全文
摘要
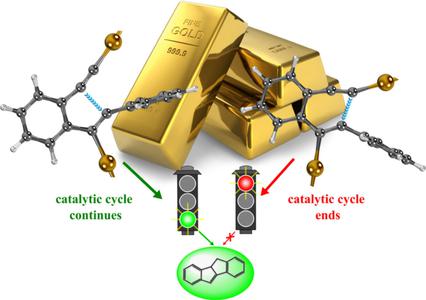
In this work, a detailed characterization was carried out of the ring-closure mechanism of EPB (1-ethynyl-2-(phenylethynyl)benzene) toward the 5-exo-dig and 6-endo-dig cyclization reactions, catalyzed by two Au–N-heterocyclic carbene (NHC) moieties. It was found that the 5-exo-dig cyclization takes place with a slightly lower activation barrier and larger exothermicity compared to that of the 6-endo-dig cyclization, in agreement with the available experimental data. A phenomenological partition (structural and electronic) for rate constants computed using transition-state theory and the reaction force analysis was used to shed light into the nature of the activation rate constant. It was found that rate constants are influenced by a strong structural component, which is larger for the 5-exo-dig cyclization due to the strain to form the five-membered ring. On the other hand, the gold activation mechanism is evidenced by a σ- and π-coordination of the Au−NHC moieties to the EPB substrate. It was found that differences in the σ-coordination arise on the reaction path for the 5-exo-dig and 6-endo-dig cyclizations. Thus, in the 6-endo-dig cyclization the σ gold–EPB interaction is weakened as a consequence of the formation of the cationic aryl intermediate, while for the 5-exo-dig cyclization this interaction was found to be favored. Furthermore, although minor changes in the Au–EPB coordination occur on the reaction path, these bonds are formally established in the TS vicinity. Results support the concerted nature of the dual gold activation mechanism.
|
Subnaphthalocyanines as Electron Acceptors in Polymer Solar ...
2018-04-10 [10.1002/chem.201800596] |
|
Formal Lossen Rearrangement/[3+2] Annulation Cascade Catalyz...
2018-04-10 [10.1002/chem.201801125] |
|
A Highly Sensitive Fluorogenic Probe for Imaging Glycoprotei...
2018-04-10 [10.1002/chem.201800790] |
|
Improvement of Photodynamic Activity of Lipid–Membrane‐Incor...
2018-04-06 [10.1002/chem.201800674] |
|
Ordered Mesoporous Titania/Carbon Hybrid Monoliths for Lithi...
2018-04-06 [10.1002/chem.201801099] |