|
~% |
|
~96% |
|
~% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |

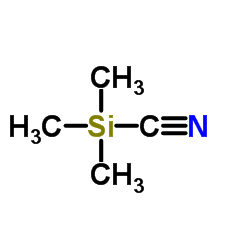
![2-[tert-butyl(dimethyl)silyl]oxyoctanal结构式](https://image.chemsrc.com/caspic/409/159680-75-8.png)
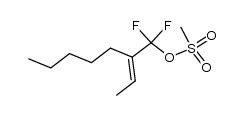
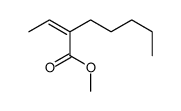
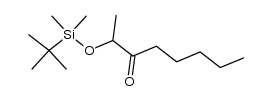
![2-{1-[(tert-butyldimethylsilyl)oxy]ethyl}-1,1-difluorohept-1-ene结构式](https://image.chemsrc.com/caspic/254/335239-75-3.png)


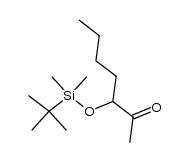
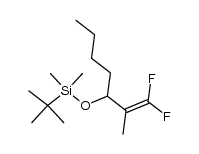
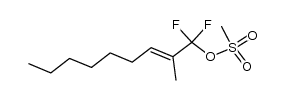
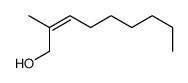
![(+/-)-3-[(tert-butyldimethylsilyl)oxy]nonan-2-one结构式](https://image.chemsrc.com/caspic/309/335239-69-5.png)
