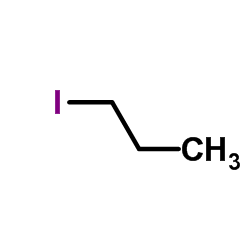
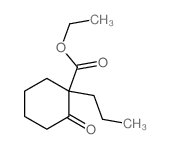
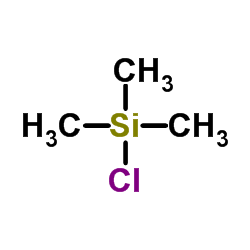
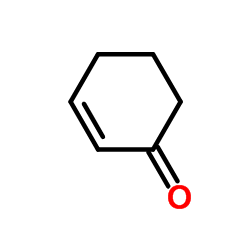
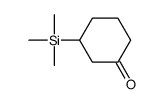
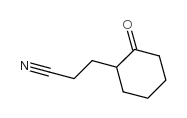
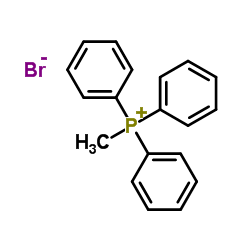
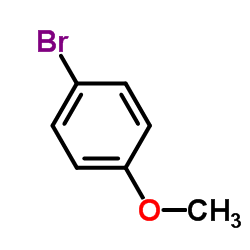
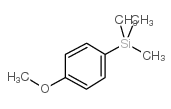
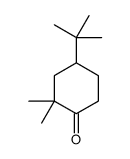
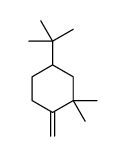
|
~68% |
|
~% |
|
~85% |
|
~66% |
|
~99% |
|
~63% |