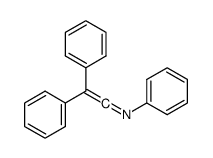
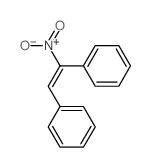
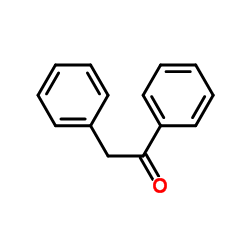
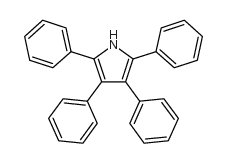
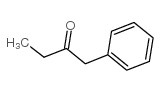
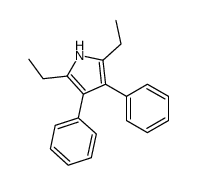
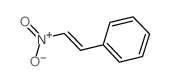
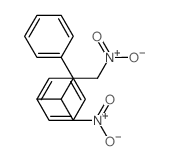
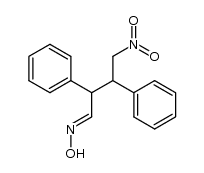
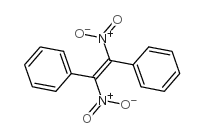
|
~60% |
|
~19% |
|
~31% |
|
~14% |
|
~55% |