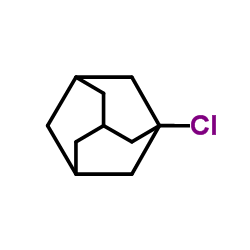
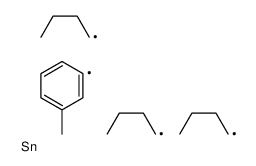
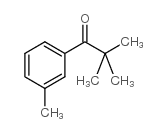
|
~61% |
|
~64% |
|
~73% |
|
~71% |
|
~68% |
|
~69% |
|
~% |
|
~61% |
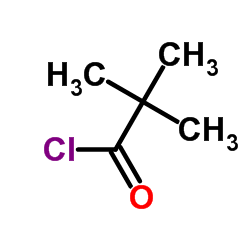
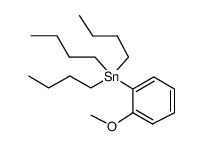
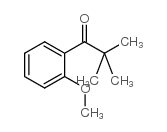

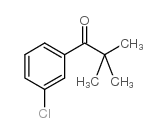
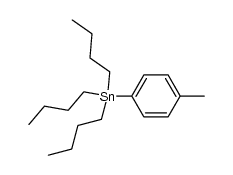


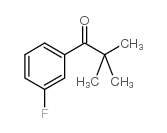
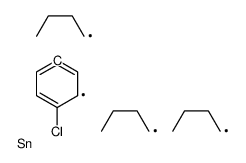
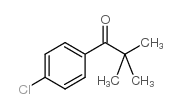
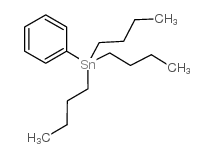
![Methanone,phenyltricyclo[3.3.1.13,7]dec-1-yl结构式](https://image.chemsrc.com/caspic/256/31919-47-8.png)