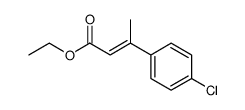
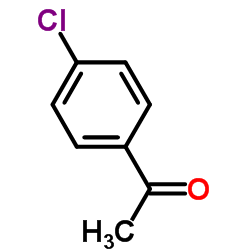
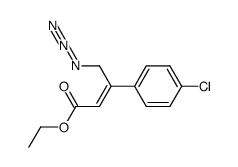
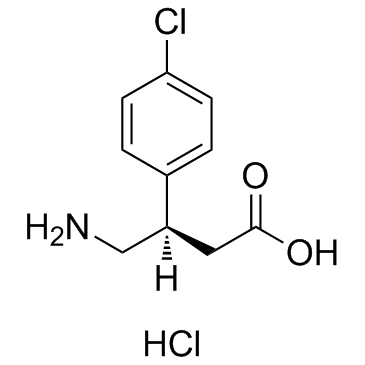
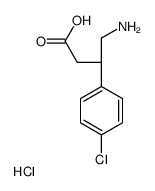
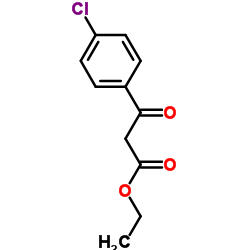
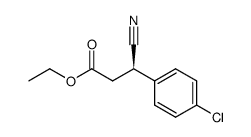
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~77% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |