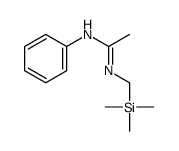
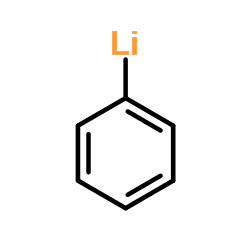
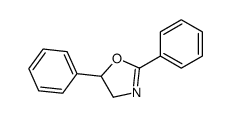
|
~91% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
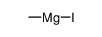
![Benzenamine, N-[[(trimethylsilyl)methyl]carbonimidoyl]结构式](https://image.chemsrc.com/caspic/130/90606-25-0.png)