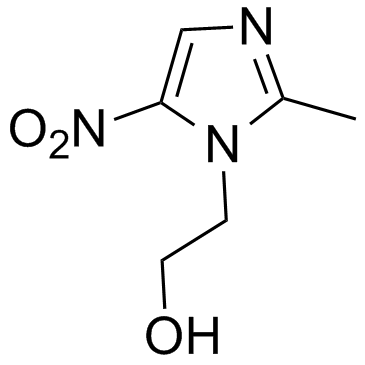
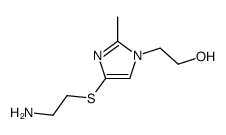
|
~65% |
|
~21% |
|
~45% |
|
~97% |
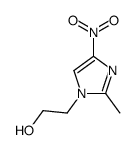
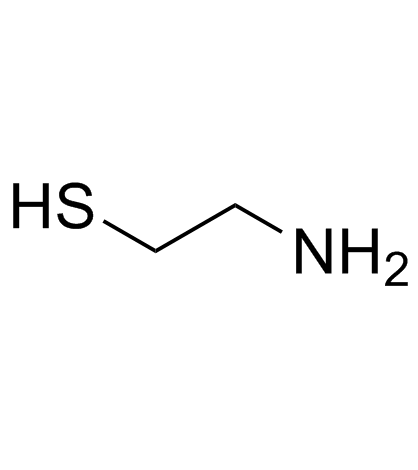
![2-[5-(2-氨基-乙基磺酰基)-2-甲基-1-咪唑]-乙醇结构式](https://image.chemsrc.com/caspic/149/78949-90-3.png)