|
~43% |
|
~14% |
|
~23% |
|
~98% |
|
~75% |
|
~% |
|
~% |
|
~81% |
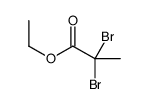
![3-[tert-butyl(dimethyl)silyl]oxybutan-2-one结构式](https://image.chemsrc.com/caspic/262/108269-33-6.png)

![1-[tert-butyl(diphenyl)silyl]oxypropan-2-one结构式](https://image.chemsrc.com/caspic/352/118171-02-1.png)

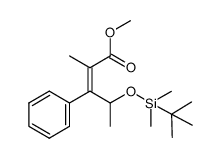
![2-[tert-butyl(dimethyl)silyl]oxy-1-phenylpropan-1-one结构式](https://image.chemsrc.com/caspic/205/133464-91-2.png)
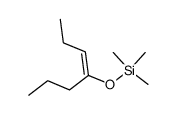

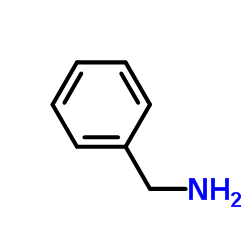
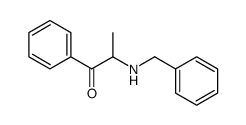
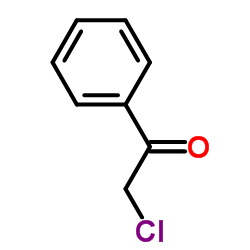
![2-[benzyl(methyl)amino]-1-phenylethanone结构式](https://image.chemsrc.com/caspic/026/33350-26-4.png)