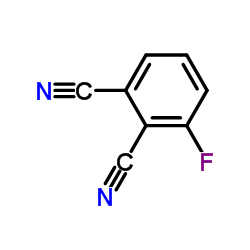
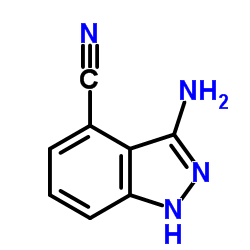
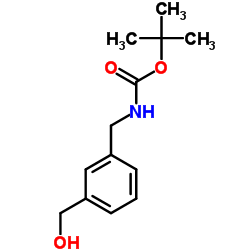
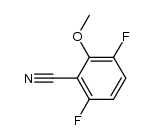
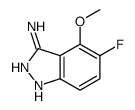
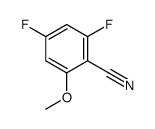
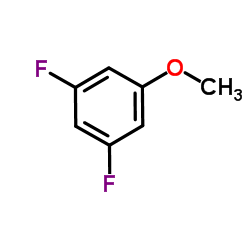
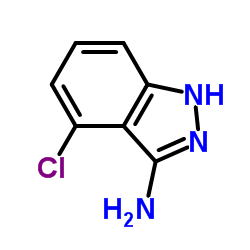
|
~85% |
|
~67% |
|
~24% |
|
~95% |
|
~% |
|
~66% |
|
~% |
|
~21% |
|
~% |