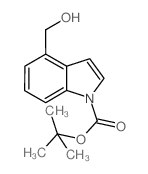
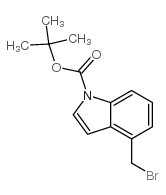
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |
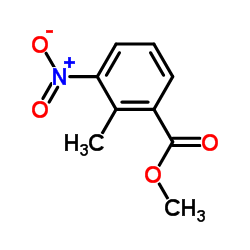
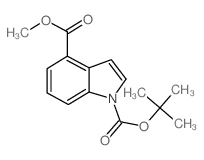
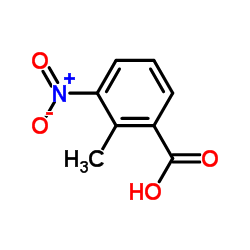
![methyl 2-[(E)-2-(dimethylamino)ethenyl]-3-nitrobenzoate结构式](https://image.chemsrc.com/caspic/469/73816-11-2.png)