|
~98% |
|
~99% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~99% |
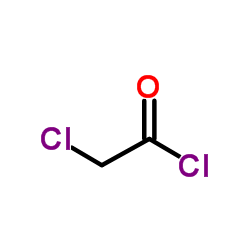
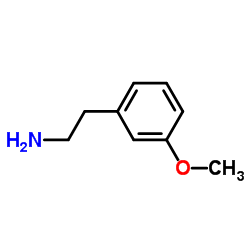
![2-氯-n-[2-(3-甲氧基苯基)乙基]乙酰胺结构式](https://image.chemsrc.com/caspic/037/34162-12-4.png)
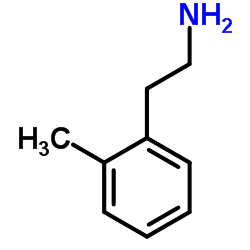
![2-氯-N-[2-(2-甲基苯基)乙基]乙酰胺结构式](https://image.chemsrc.com/caspic/112/141463-66-3.png)
![1-Methyl-2-[(E)-2-nitrovinyl]benzene结构式](https://image.chemsrc.com/caspic/122/28638-59-7.png)
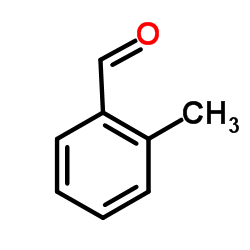

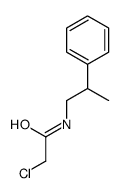
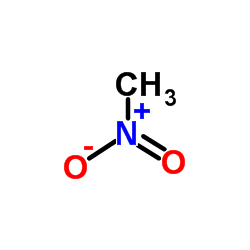
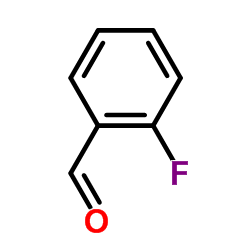
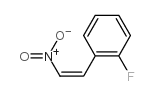

![2-Chloro-N-[2-(4-methoxyphenyl)ethyl]acetamide结构式](https://image.chemsrc.com/caspic/093/17639-50-8.png)