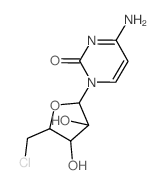
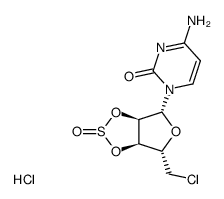
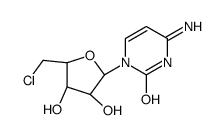
|
~85% |
|
~% |
|
~% |
|
~78% |
|
~% |
![6H-Furo[2',3':4,5]oxazolo[3,2-a]pyrimidin-3-ol,2-(chloromethyl)-2,3,3a,9a-tetrahydro-6-imino-, hydrochloride (1:1),(2S,3S,3aS,9aR)结构式](https://image.chemsrc.com/caspic/143/32659-29-3.png)